STELFONTA® (tigilanol tiglate injection) Pet Owner Survey
- See why STELFONTA is the option pet owners prefer and other veterinarians continue to use it.
STELFONTA® (tigilanol tiglate injection) Information
- Information on STELFONTA, the treatment process, clinical study information, and a case study
STELFONTA In Practice
- How to use STELFONTA in your hospital - practical tips and useful information
Technical Monograph
- Scientific information on Mast Cell Tumors and STELFONTA treatment, studies, and how to use STELFONTA
When To Assess Response?
- When and how to assess response after treatment with STELFONTA
Client Information Sheet
- A document to share with clients that have selected STELFONTA
Clinician's Casebook
- Cases from several veterinary hospitals
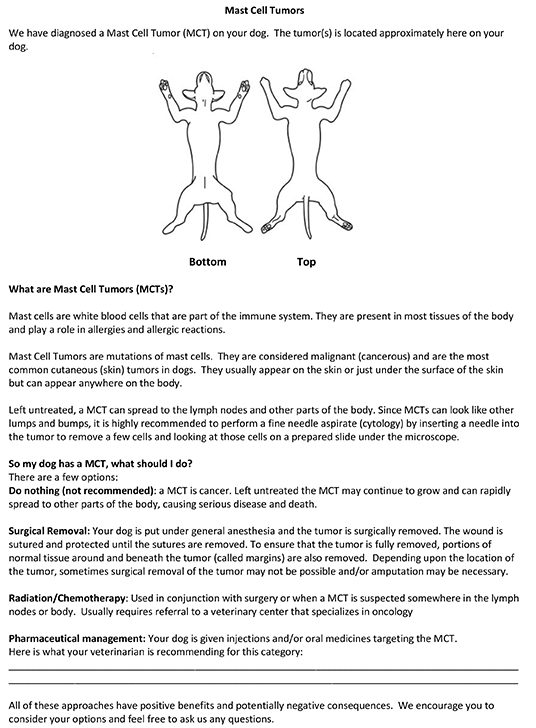
What is a MCT?
- Educational document for pet owners on mast cell tumors and available treatment options
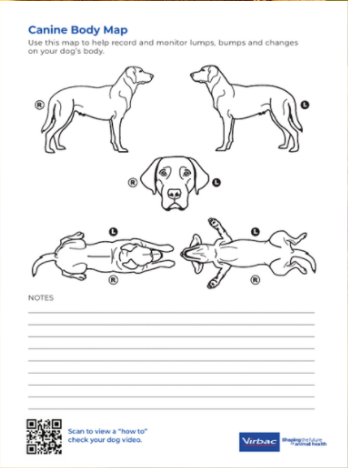
Canine Body Map
- Handout for the medical team to share with pet owners to help track lumps or bumps, including home skin check instructions
Medication Schedule Tracker
- Pet owner handout for tracking mandatory concomitant medication administration
Email Support Program
- Have your pet owners sign up for the STELFONTA email support program once they have scheduled their treatment date
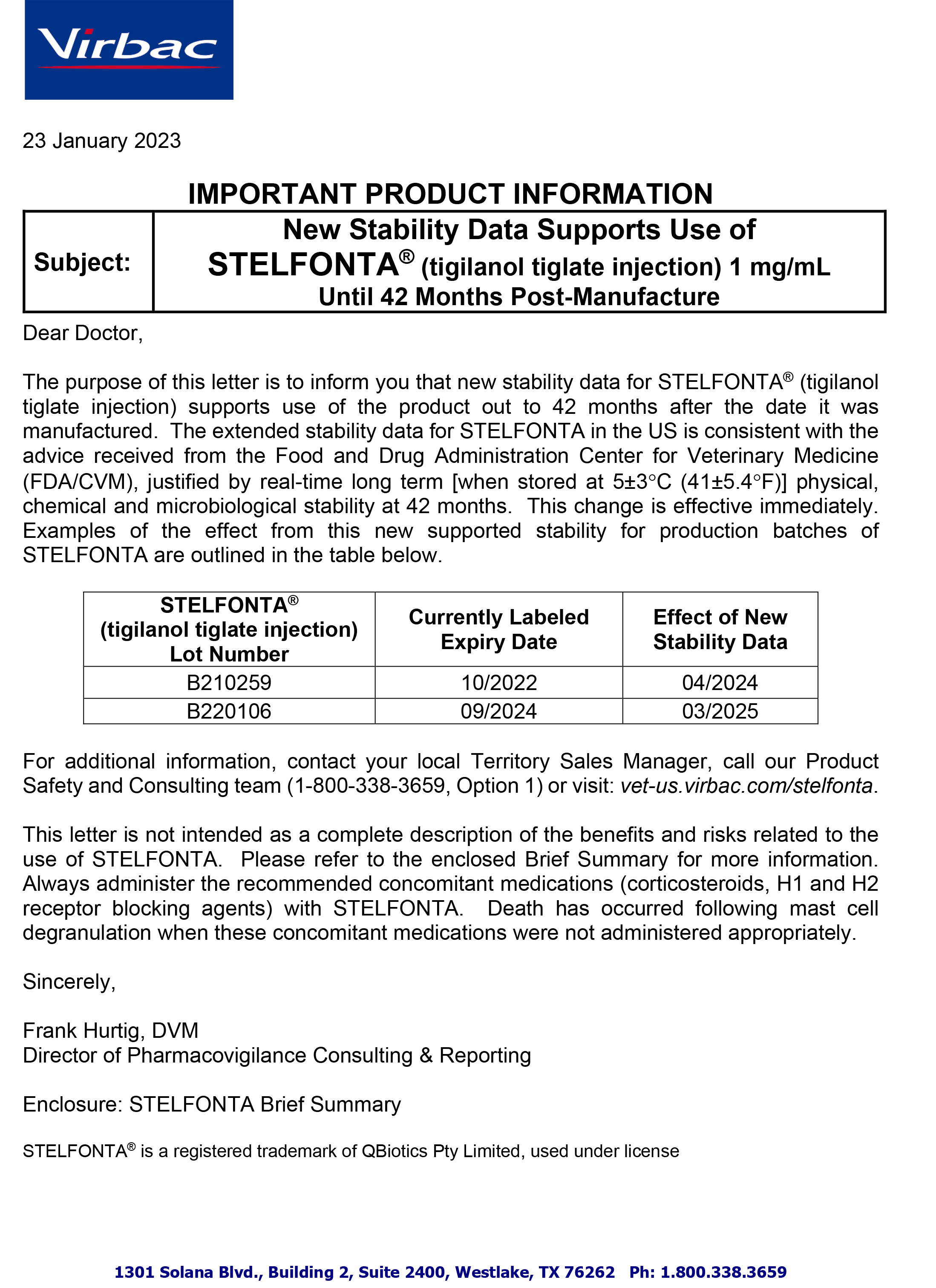
Stelfonta Expiry Letter 2023
- Expiration extension information
Safety Data Sheet
- For SDS records
IMPORTANT SAFETY INFORMATION
Accidental self-injection of STELFONTA® (tigilanol tiglate injection) may cause severe wound formation. To decrease the risk of accidental self-injection, sedation of the dog may be necessary. In dogs, do not inject STELFONTA into subcutaneous mast cell tumors located above the elbow or hock. Formation of wounds, possibly extensive, is an intended and likely response to treatment with STELFONTA along with associated swelling, bruising and pain; these wounds are expected to heal. Some of these cases resulted in amputation. Appropriate pre- and post-treatment medications must be given, including a corticosteroid plus blocking agents for both H1 and H2 receptors, in order to decrease the potential for severe systemic adverse reactions, including death, from mast cell degranulation. For full prescribing information, contact VIRBAC at 1-800-338-3659 or view the Product Insert.